The 8 trends for the Healthcare sector in 2022 are:
- The pandemic could end but COVID-19 will still be on the picture
- Precision medicine to drive the era of genomic profiling, biomarkers, synthetic medicine, and miniaturized robotics
- Biotechnology is expected to speed up pharmaceutical testing and drugs approval
- Healthcare is moving to the cloud
- Artificial Intelligence and the Internet of Medical Things will render unprecedented accuracy
- Telemedicine is here to stay
- Biosimilars will continue to make high-cost medicines more accessible
- Health to take a more holistic approach with mental wellbeing being a priority
Mostly focused on technology advancements and the pursuit of a more balanced and integral conception of health, keep reading to learn what these 8 trends are all about.

As for the past two years, the coronavirus has been both the protagonist and the screenwriter of what seems to be an endless film. So when will it end?
Although more than half of the world is already fully vaccinated1, there are still significant inequality gaps regarding the vaccine roll-out (to which is to be added the growth of strong anti-vaccine sentiment), leaving a worrisome 40,16% of the population still unprotected to the virus.
Director-General of the World Health Organization (WHO) since 2018, Tedros Adhanom Ghebreyesus, believes 2022 must be the last year of the pandemic, as WHO projections show that vaccine supplies should be sufficient to vaccinate the entire global adult population and to give boosters to high-risk populations by the first quarter of 20222.
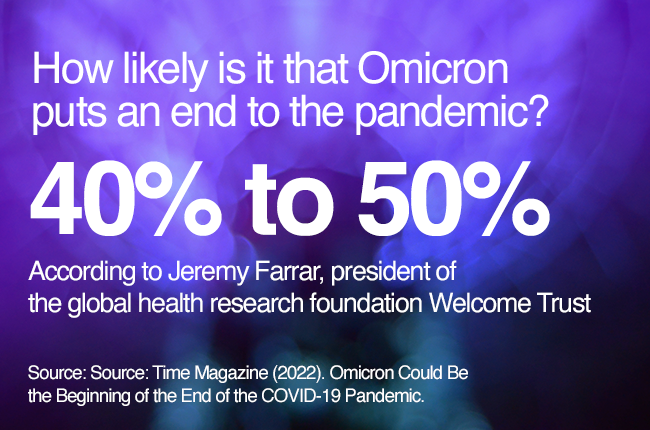
However, the lightspeed spread of Omicron in recent months has fueled uncertainty and fears that the pandemic will continue to drag on.
Experts opinion is divided into two sides: one, more optimistic, believing Omicron could actually be the beginning of the end, since it has become the dominant variant and it carries a much less severe infection, which, in time, could make COVID-19 “a more manageable endemic disease like the seasonal flu.”3
And the other side, more cautious, avoids underestimating the unpredictability of the virus, saying it could keep changing into potentially more harmful variants and suggesting governments should increase surveillance and efforts4.

Precision medicine’s goal is to offer the best possible options for each individual given their environment, genetics and habits, so naturally it has seen their practices bolstered by evolving technology and big data.
It also facilitates preventative medicine, allowing the identification of patients with a genetic predisposition for a particular disease to enable pre-emptive treatment.
According to Mathys & Squire LLP, the global personalized medicine market is forecasted to grow to $717 billion by 20255.
Patients are experiencing the wonders of precision medicine in the form of immunotherapy for cancer, better insulin dosing for diabetics, as well as drugs specially formulated for cystic fibrosis patients with particular gene mutations.
Personalized medicines cover a wide range of activities, from AI-powered imaging biomarkers6 and tools of the size of a grain of salt7 to advanced therapy medicinal products (ATMPs) such as stem cells and CAR-T cells. In fact, ATMPs products are expected to increase markedly from the current level of around a third of pharmaceutical pipelines over the next decade.
Ken Mills, CEO of Regenxbio, maker of viral vectors for gene therapy, forecasts that upcoming launches and the continued global rollouts of previously approved products will bring cell and gene therapy “more into the public consciousness in 2022, and it will just keep building.”8

Just 11 months after the SARS-CoV-2 was first identified, three vaccines were approved, which set a record and challenged drug development standards (before, the fastest vaccine ever took four years to be developed)9.
Yes, the battle against COVID-19 gathered the world’s efforts and resources, but this milestone was also possible because the medical community dared to break molds. The success of the vaccines exposed inefficiencies within drug regulatory processes and demonstrated that we have the technology to speed up approval rates, that is to say, to deliver effective treatments to patients in less time without compromising safety.
Biotechnology data is being used to prop pharmaceutical testing up by allowing simulations of interactions between medicines and the human body, as well as predicting patient response to medications or analyzing diagnostic results, rather than having to rely on costly and time-consuming human trials for every stage of the process.
FDA’s Real-Time Oncology Review program is an example of a drug testing and review process that has been greatly accelerated by the inclusion of biotech data, specifically due to its potential to accurately predict testing outcomes of trials of novel cancer treatments10.
In 2022, laboratories and healthcare companies are expected to increase their investments in technologies, workflows, and change management capabilities specifically designed to shorten development cycles. David Dorsey, Director of Janssen R&D, commented on the subject11:
“[In 2022] we expect accelerated approval will be back on the docket for consideration by Congress as it goes into the user fee reauthorizations next year.”
Furthermore, The Food and Drug Administration will announce a new commissioner in the 1Q of the year, and the name of Rober Califf, former FDA head (2016-2017), has been nominated. Dr. Califf is known as an innovative clinical trialist, who advocates for technology and digitalization, so, bets are, his office could prioritize the modernization of clinical trials and regulatory processes in the US12.

In July 2021, Amazon HealthLake became officially available. HealthLake is a HIPAA-eligible service for health care and life sciences organizations to store and analyze their data, using machine learning to process and make sense of unstructured data, as a promise to provide “a holistic view of patient health.” Nothing validates more a booming trend than the biggest tech companies getting involved – healthcare is unstoppably transitioning to the cloud13.
Unifying information in the cloud and making it accessible to shared networks of doctors, laboratories, research and health insurance companies, laboratories, patients and pharmacies alike will create a seamless line of communication between all healthcare-related entities.
Cloud-based platforms are making novel systems like patient portals possible, offering interoperability and a secure way for important information to be transmitted quickly and easily from anywhere, at any time.
According to Vantage Market Research’s recent report, Healthcare Cloud Computing Market to reach US $ 70 billion by 202814.

Artificial Intelligence (AI) devices have moved from scheduling and administrative tasks to learn patterns to recognize how a person will metabolize drugs, analyze tests and medical records to track the effects of different therapies on groups of patients over time, and to identify abnormalities in imaging15.
So far, AI has worked as a kind of assistant for specialists, but in 2022 advancements into having AI devices could mean that they are equally as important as professionals—they’ve even proven to sometimes be keener than the human eye.
Using AI to determine if someone will respond positively to a given treatment is particularly useful in oncology and neurology, where researchers can help doctors select treatments specific to a patient’s needs.
Hc1 chairman and CEO, Brad Bostic, in a discussion with Brian Patty, MD, CMIO, Medix Technology, during the Twenty-First Population Health Colloquium, said16:
“If you think about the more tangible way to describe what AI can bring to bear is the digital twin. What we need to have is the computational model of every single individual patient that can be tested in a digital way before the actual human being receives the treatment or gets the kind of diagnostic that’s really invasive or has some kind of radical procedure.”
Likewise, the Internet of Medical Things (IoMT) is the technology that makes possible the accuracy of wearable patient devices, remote patient monitoring (RPM) of people with chronic or long-term conditions, personal emergency remote systems (PERS), and the others segments17 of the industry that includes point-of-care devices and asset management monitors.

Although telemedicine has been available for several years, the pandemic and the need for physical distancing was responsible for a major acceleration of virtual care over the last two years —a market that could reach $250 billion, according to a July, 2021 McKinsey & Company report18.
However, a hybrid model balancing virtual and in-person meetings is to be expected:
Patients will be encouraged to do more initial consultations via video call, and depending on their diagnosis prescriptions could be sent through mail or a following face-to-face appointment may be scheduled.
Besides, when it comes to acute and chronic disease management, virtual care programs allow doctors to monitor patients remotely using sensors that track vital signs, health records and other personal information.
Telemedicine is here to stay, and it will improve progressively to reduce cost and time for patients and doctors, as well as to create a more accessible healthcare system for everyone.

2021 was a good year for biosimilars —especially with oncological products like Avastin, Herceptin and Rituxan having their Biosimilar Preferred Products approved19— and 2022 will follow the lead.
But what are Biosimilars? According to the FDA: “a biosimilar is a biologic (Medications from Living Organisms) that is highly similar to, and has no clinically meaningful differences from, another biologic that’s already FDA-approved (referred to as the reference product or original biologic)20.”
Drugs created from natural sources usually translate into high-cost medicines, so Biosimilars represent a less expensive alternative. Experts consider Biosimilars will play a key role in the future of America’s healthcare system, helping patients access critical treatments21. Currently, the State is encouraging biosimilars production and use from a policy perspective22.
Here is a freshly-baked report on Projected US Savings From Biosimilars.
While precision medicine and AI-driven technologies gain accuracy by reducing the human factor, a more humane and holistic approach to health has also gained momentum.
Mental health awareness increased by the pandemic: a study23 published in October 2021 found that “two COVID-19 impact indicators, specifically daily SARS-CoV-2 infection rates and reductions in human mobility, were associated with increased prevalence of major depressive disorders and anxiety”.
However, this helped reduce stigma around seeking care for mental health, which is aligned with Healthy People 2030, a national project that has set data-driven goals to improve the health and well-being of people in the United States.
Healthy People 2030 takes into consideration Social Determinants of Health (SDOH): the conditions in the environments where people are born, live, learn, work, and age that affect a wide range of health, functioning, and quality of life outcomes and risks24.
Although the middle point between “high-tech” and “high-touch” is far from being reached, an industry formerly seen as distant and unreachable could be closer than ever to finally live to its promise: looking for people’s well-being above all conditions.
Sources
- Our World In Data. (2022). Coronavirus (COVID-19) Vaccinations [January 17]. [Online]. Available at: https://ourworldindata.org/covid-vaccinations?country=OWID_WRL
- Collis, H. (2021). WHO forecasts coronavirus pandemic will end in 2022. Politico. [December 22]. [Online]. Available at: https://www.politico.eu/article/who-forecasts-coronavirus-pandemic-will-end-in-2022/.
- France 24. (2022). Europe could be headed towards end of pandemic after Omicron, says WHO. [January 24]. [Online]. Available at:https://www.france24.com/en/europe/20220123-europe-could-be-headed-towards-end-of-pandemic-after-omicron-says-who
- Park, A. (2022). Omicron Could Be the Beginning of the End of the COVID-19 Pandemic. Time Magazine. [January 26]. [Online]. Available at: https://time.com/6141679/omicron-end-covid-19/
- Gregson, A. (2022). Title of article. Marthys & Squire. [January 24]. [Online]. Available at: https://www.mathys-squire.com/insights-and-events/news/ip-trends-in-2022-personalised-medicine-and-advanced-therapy-medicinal-products/
- Brainomix. (2022). [Online]. Available at: https://www.brainomix.com.
- News Round. (2021). Scientists develop a camera that’s ‘the size of a grain of salt! [December 2]. [Online]. Available at: https://www.bbc.co.uk/newsround/59483432
- Weintraub, A. (2021). 2022 forecast: Cell, gene therapy makers push past regulatory, payer hurdles to set up high hopes for next year. Fierce Pharma. [December 22]. [Online]. Available at:https://www.fiercepharma.com/pharma/cell-and-gene-therapy-makers-push-past-regulatory-payer-hurdles-to-set-up-high-hopes-for
- McKinsey & Company. (2021). Fast-forward: Will the speed of COVID-19 vaccine development reset industry norms? [May 13]. [Online]. Available at: https://www.mckinsey.com/industries/life-sciences/our-insights/fast-forward-will-the-speed-of-covid-19-vaccine-development-reset-industry-norms
- Marr, B. (2021). The 5 Biggest Biotech Trends In 2022. Forbes. [December 8]. [Online]. Available at: https://www.forbes.com/sites/bernardmarr/2021/12/08/the-5-biggest-biotech-trends-in-2022/?sh=7c3ede8f380f
- Schneider, M. (2021). FDA’s accelerated approval program: Is change on the way? Regulatory Affairs Professionals Society (RAPS). [October 11]. [Online]. Available at: https://www.raps.org/news-and-articles/news-articles/2021/10/fdas-ccelerated-approval-program-is-change-on-the.
- Gardner, J. (2022). 5 questions facing the FDA in 2022. Biopharma Dive. [January 11]. [Online]. Available at: https://www.biopharmadive.com/news/fda-2022-outlook-questions-trends-califf/616849
- Business Wire. (2021). AWS Announces General Availability of Amazon HealthLake. [July 15]. [Online]. Available at: https://www.businesswire.com/news/home/20210715005761/en/AWS-Announces-General-Availability-of-Amazon-HealthLake
- Vantage Market Research . (2022). Healthcare Cloud Computing Market to reach US $ 70 billion by 2028 – Global Insights on Size, Trends, Key Leaders, COVID-19 Impact Analysis, Regulatory Landscape, and Growth Opportunities: Vantage Market Research. [January 4]. [Online]. Available at: https://www.globenewswire.com/news-release/2022/01/04/2360474/0/en/Healthcare-Cloud-Computing-Market-to-reach-US-70-billion-by-2028-Global-Insights-on-Size-Trends-Key-Leaders-COVID-19-Impact-Analysis-Regulatory-Landscape-and-Growth-Opportunities-V.html
- Gupta, A. (2021). The Future Of Health: Three Healthcare Trends For 2022. Forbes. [December 14]. [Online]. Available at: https://www.forbes.com/sites/forbesbusinesscouncil/2021/12/14/the-future-of-health-three-healthcare-trends-for-2022/
- VanDenBoom, L. (2021). 10 Healthcare Industry Trends to Watch in 2022. Hc1. [December 10]. [Online]. Available at: https://www.hc1.com/blog/10-healthcare-trends-2022/
- Alliance of Advanced BioMedical Engineering. (2017). Internet of Medical Things Revolutionizing Healthcare. [Online]. Available at: https://aabme.asme.org/posts/internet-of-medical-things-revolutionizing-healthcare
- Morris, P. (2022). Top 5 Healthcare Tech Trends Poised for Growth in 2022. Entrepreneur. [January 5]. [Online]. Available at: https://www.entrepreneur.com/article/402155
- Highmark. (2021). Medical Policy Update [April]. [Online]. Available at: https://content.highmarkprc.com/Files/Region/hbcbs/NewsletterNotices/MPU/April%20WESTERN%20FINAL.pdf
- U.S. Food and Drug Administration. (2021). Biosimilar and Interchangeable Biologics: More Treatment Choices. [October 12]. [Online]. Available at: https://www.fda.gov/consumers/consumer-updates/biosimilar-and-interchangeable-biologics-more-treatment-choices
- Baldetti, J. (2021). What’s Next for the Biosimilars Market in the U.S.? Managed Healthcare Executive. [November 22]. [Online]. Available at: https://www.managedhealthcareexecutive.com/view/what-s-next-for-the-biosimilars-market-in-the-u-s-
- Congress Government. (2021). S.164 — 117th Congress (2021-2022). [April 23]. [Online]. Available at: https://www.congress.gov/bill/117th-congress/senate-bill/164#:~:text=Advancing%20Education%20on%20Biosimilars%20Act%20of%202021%20This,address%20the%20prescribing%20of%20biological%20products%20and%20biosimilars.
- The Lancet. (2021). Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. [October 8]. [Online]. Available at: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)02143-7/fulltext
- Health Government. Social Determinants of Health. [Online]. Available at: https://health.gov/healthypeople/objectives-and-data/social-determinants-health


